Chemists are working with the U.S. Army to produce uniforms that can rapidly break down toxic substances and protect soldiers from chemical weapons.

IN OMAR FARHA’S lab at Northwestern University, the chemist and his team are working on an unusual craft project in collaboration with the United States Army. They mix powders and liquids into a paint-like consistency, dip swatches of cotton fabric into the liquid, and then leave the beige cloth out to dry. Through this process, they are creating fabrics that can rapidly neutralize some of the deadliest poisons known to humankind: nerve agents.
These fabrics are the latest development in a 10-year effort to design military uniforms that better protect wearers against chemical weapons. Farha’s cloth specifically destroys the nerve agents VX and soman, also known as GD, which is a more toxic relative of sarin. These chemicals disrupt the human central nervous system—essentially stopping the body’s cells from communicating with each other. They can also kill swiftly without needing to be ingested. In 2017, for example, Kim Jong-nam, the half-brother of North Korean dictator Kim Jong-un, was assassinated in the Kuala Lumpur airport by two women who allegedly smeared VX on his face. Kim died within two hours of exposure.
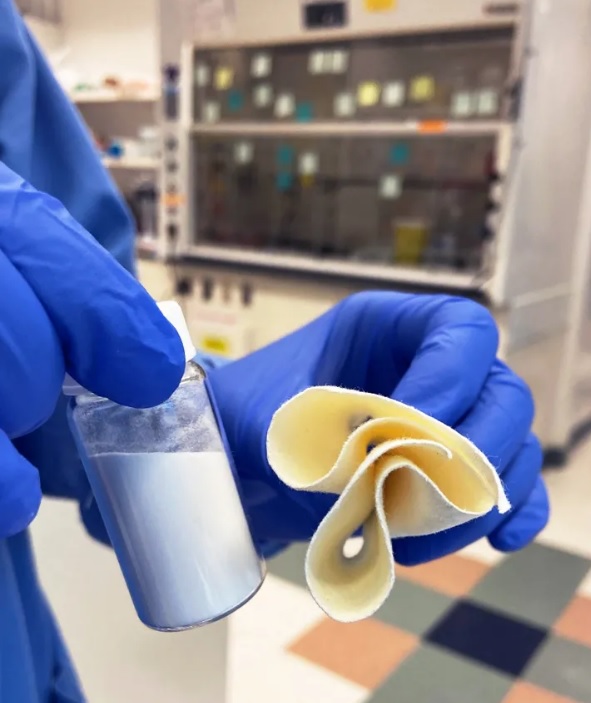
Currently, US soldiers have uniforms that absorb nerve agents, but don’t destroy them. The goal is to make a uniform that can do both, says chemist Jared DeCoste, a researcher with the US Army who was not involved with the work. DeCoste is developing similar fabrics that neutralize mustard gas, a chemical weapon that is not a nerve agent but can severely burn the skin, eyes, and respiratory tract. His group has already incorporated this anti-mustard technology into prototype gas masks.
Despite their nastiness, chemists can neutralize these nerve agents easily enough if they pour them into beakers of solution. Regular water breaks down these toxins slowly over days, but chemists can add specific materials called catalysts that speed up the reaction time to minutes.
Farha’s challenge was to orchestrate this reaction on dry fabric. His team coated the fabric with one key ingredient: a crumpled crystalline molecule called MOF-808 (MOF rhymes with “cough”). This molecule essentially harvests water from ambient air. Water vapor likes to condense onto MOF-808 molecules because of their shape and chemical properties. When MOF-808 makes contact with a nerve agent, the water attached to the molecule breaks down the toxin, while zirconium atoms that recur throughout MOF-808’s crystal serve as the catalyst, accelerating the nerve agent’s breakdown. As long as the fabric is worn in a place where the humidity level is at least 30 percent, it can collect enough water to break down nerve agents in minutes.

Farha’s team tested the fabric’s efficacy in conditions that would be fairly realistic for an active duty soldier, dirtying it with diesel and artificial sweat, for example. These contaminants did not significantly lower its performance. In fact, sweaty fabric performed better than clean fabric—probably because of the extra water.
MOF-808 belongs to a larger class of molecules known as metal-organic frameworks, which chemists have begun to use to more precisely control chemical reactions. Broadly speaking, these frameworks consist of metal atoms linked to chains of organic molecules to form cage-like crystalline structures, which can be put in a powder form. Chemists can tune the properties of these structures to attract specific molecules like water. You can think of these molecules as being like folded-up accordions: extensive surfaces fitted into compact spaces. This expansive surface area allows MOF-808, for example, to collect a lot of water relative to its size. Just a dime-sized dollop of metal-organic frameworks comprises about two football fields’ worth of surface area, says chemist Yuzhang Li of Stanford University.
Once these molecules get stuck inside the cage, chemists can then direct them to interact in a desired way. Researchers have designed more than 50,000 types of metal-organic frameworks, each a potential stage for a particular set of chemical reactions. In particular, chemists want to use these customized cages for storing gases—perhaps for trapping carbon dioxide produced at a coal plant, or storing hydrogen gas for fuel cells.
Farha’s fabric coating also uses a polymer called polyethylenimine, which glues the metal-organic framework to the cloth evenly. But achieving this uniform layer was a bit of a fluke. Chemists don’t have a detailed picture of how a metal-organic framework attaches to a surface, so they’re still not clear on the best way to make the molecules stick.
Li has developed a technique for photographing metal-organic frameworks that could help answer this question. In Li’s method, he triggers the metal-organic framework to undergo a chemical reaction, and then plunges it into liquid nitrogen. Then, he photographs the framework under a microscope. The method, known as cryogenic electron microscopy, is adapted from a similar technique in biology. It freezes the chemical reaction in time, allowing a chemist to study the reaction frame by frame. Li’s team used the technique to image a carbon dioxide molecule trapped inside a metal-organic framework. These more detailed images could lead researchers to design frameworks that perform specific chemical reactions better, says Li.

Now that Farha’s fabric performs the desired chemical reaction, his team will begin to consider its wearability. For soldiers to make use of the fabric’s extra protection, his team now needs to make it function as a piece of clothing. For Farha, that means answering questions like whether or not the coating flakes off, and if the fabric is breathable.
Basic research projects like Farha’s have now laid most of the scientific groundwork needed to make these uniforms, he says. While researchers have to tweak the designs, run more tests, and figure out how to scale up production, Farha thinks the military will be able to adopt these chemically-sophisticated uniforms in a few years.
But the power of metal-organic frameworks lies well beyond just military uniforms. In particular, they allow chemists the freedom to design molecules for a desired application. Chemists can mix and match metal atoms with different organic compounds to form customized shapes—kind of like playing with the world’s tiniest Legos. “You have the entire periodic table of elements to choose from,” says Farha. Poison-proof uniforms are only the beginning.
 iTechBahrain Information Technology Digital Marketing Web & Mobile Development Services
iTechBahrain Information Technology Digital Marketing Web & Mobile Development Services